Drawing the Lewis Structure for SiCl4
Viewing Notes:
- SiCl4 is similar to the SiF4 Lewis structure.
- The Lewis structure for SiCl4 has 32 valence electrons available to work with.
Valence electrons contribute to chemical reactions and bonding within the structure of a material and determine its electrical properties. Figure 2: Illustration of the Bohr model of the silicon atom. Maximum number of valence electron is 8. An atom is stable if it has 8 valence electrons.
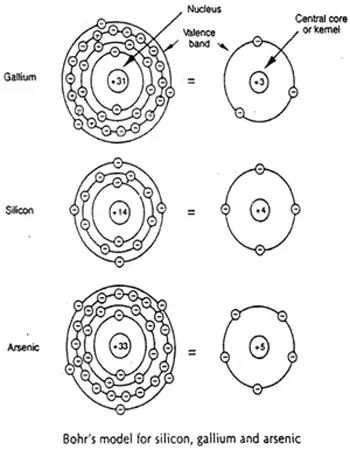

There are two ways to find the number of valence electrons in Silicon (Ca). The first is to use the Periodic Table to figure out how many electrons Silicon h. How maney valence electrons does it have its right? Its electron configuration In corn rotation, we can see outside the core here, silicon has four valence electrons and for C which sub shells hold the villains electrons. So we outside the core you balance electrons are located in the three s and three p sub shells.
See the Big List of Lewis Structures
Si Valence Electrons Worksheet
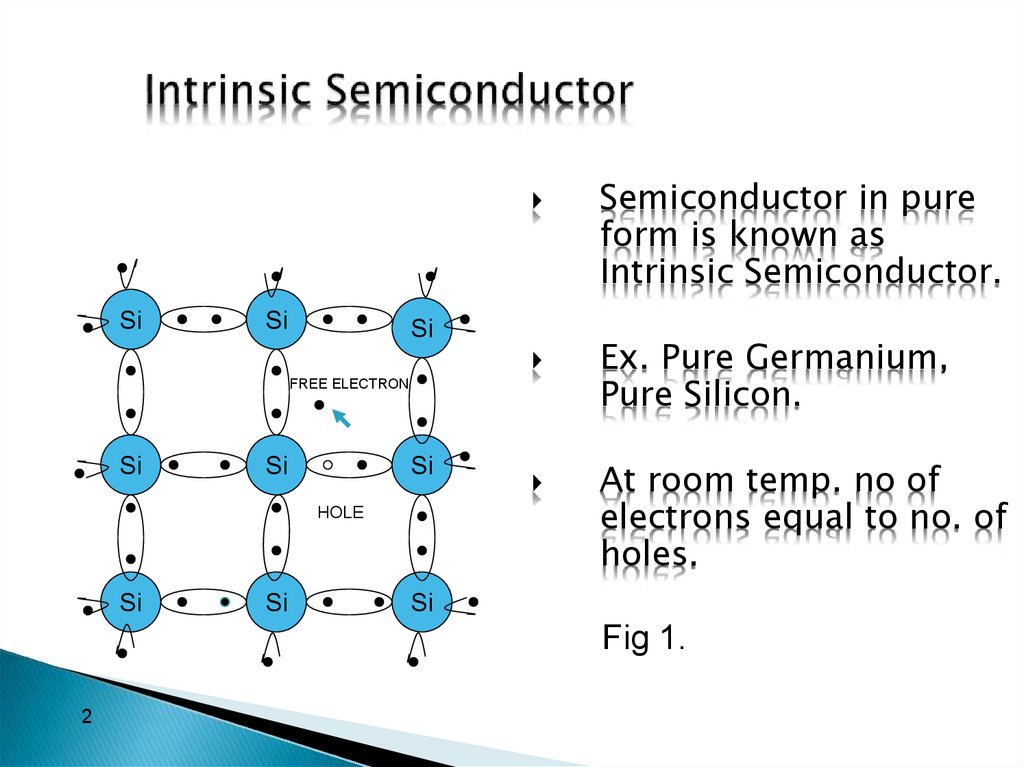
Silicon Valence Electrons Level
Transcript: Hi, this is Dr. B. Let's do the SiCl4 Lewis structure. Si is in group 4 or 14 on the periodic table, so it has 4 valence electrons. Cl, group 7 or 17 has 7. We've got four Cl's there, so if we multiply that all together we've got 28 plus 4: 32 total valence electrons. We'll put the Si at the center. It is the least electronegative. Then put the Chlorines on the outside.
So now we have 32 valence electrons. We'll put two between the atoms to form chemical bonds. There we've used eight. And then around the outside: 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32. Thirty-two valence electrons. OK, let's see if we have octets. So Chlorine needs eight valence electrons. That Chlorine has eight, as does that, that, and that. And in the center, Si has eight valence electrons, as well.
Element Si Valence Electrons
So we've used all 32 valence electrons. Each of the atoms has an octet or full outer shell, and that is the Lewis structure for SiCl4. This is Dr. B., and thanks for watching.
